Oseltamivir Pediatric Dosing Calculator
Calculate Pediatric Oseltamivir Dosage
Select child's weight and formulation type to determine the correct dose.
When dealing with seasonal flu or a sudden pandemic, getting the right antiviral to the right patient at the right time can save lives. Oseltamivir is an oral neuraminidase inhibitor that shortens the course of influenza and reduces complications. But in clinics that lack reliable electricity, cold storage, or steady drug supplies, delivering those tablets or liquid doses becomes a real puzzle.
What is Oseltamivir and Why It Matters
Oseltamivir belongs to the class of Neuraminidase Inhibitors, drugs that block a viral enzyme essential for influenza to spread from cell to cell. In the 2009 H1N1 pandemic, the World Health Organization (WHO) highlighted oseltamivir as a cornerstone of the global response, placing it on the WHO Essential Medicines List. Its oral formulation makes it easier to distribute than inhaled options, yet the very simplicity of a pill hides a web of logistical hurdles in low‑resource environments.
Standard Dosing Regimens and Their Implications
For otherwise healthy adults, the usual regimen is 75 mg twice daily for five days. Children receive weight‑based doses, often requiring a pediatric suspension that must be mixed accurately. Pregnant women are also eligible, but they need careful counseling about timing and potential side effects. Each of these scenarios assumes the presence of calibrated measuring devices, reliable patient education, and a supply chain that can keep the drug within its shelf‑life.
Infrastructure Gaps: The Cold‑Chain Misconception
Unlike some vaccines, oseltamivir is stable at room temperature up to 25 °C (77 °F). However, many remote clinics lack climate‑controlled storage, leading to temperatures that exceed the stability window during hot seasons. When temperatures rise, the drug’s potency can degrade, turning a life‑saving tablet into a sub‑therapeutic dose. In places without reliable thermometers, staff often have no way to verify that the drug remains effective.
Supply Chain and Stock Management Challenges
Even in well‑funded health systems, the journey from manufacturer to bedside involves multiple hand‑offs: national procurement agencies, central medical stores, regional distributors, and finally the clinic. Each step is a point where stock can be lost, delayed, or damaged. In resource‑limited settings, the lack of digital inventory tools means stock‑outs are discovered only when patients arrive and the medication is unavailable. The result is a cascade of missed treatment windows, especially since oseltamivir is most effective when started within 48 hours of symptom onset.
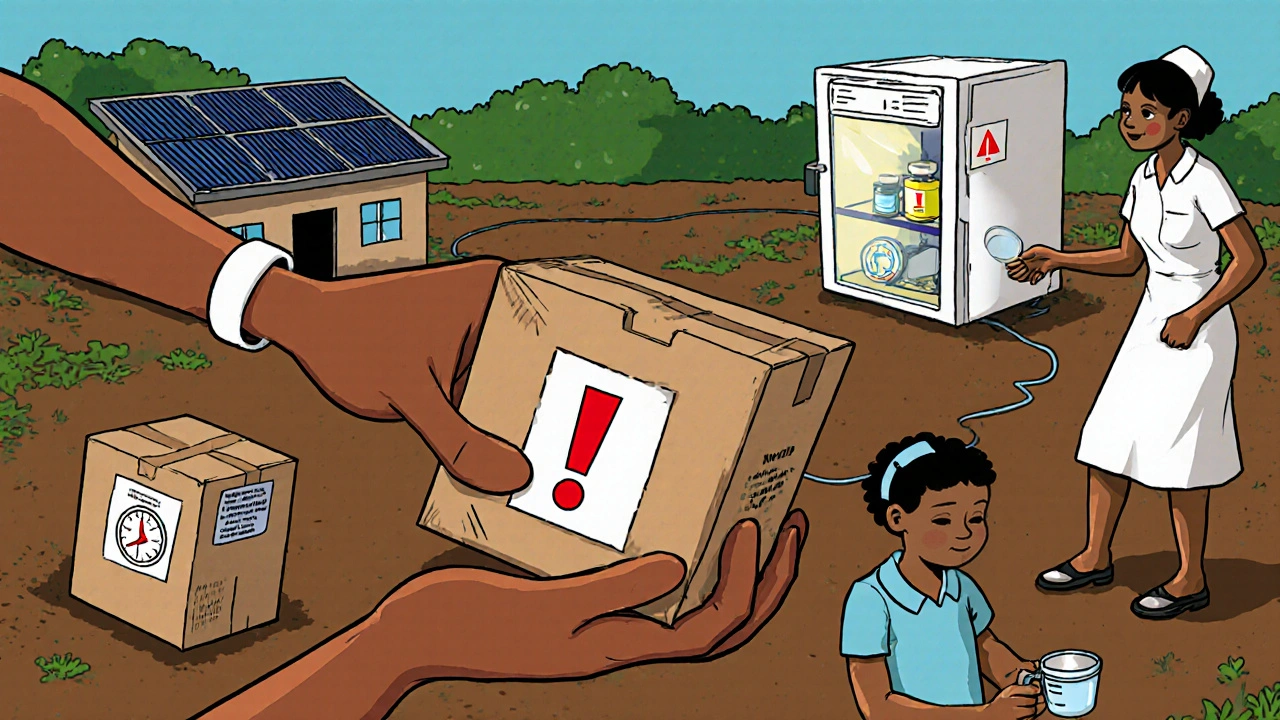
Diagnostic Limitations and Timely Initiation
Rapid diagnostic tests (RDTs) for influenza can confirm infection in under 30 minutes, but they require refrigerated storage and quality control-both scarce in many low‑resource clinics. Without a reliable test, clinicians must rely on clinical judgment alone, which can lead to over‑prescribing (wasting limited supplies) or under‑prescribing (missing the therapeutic window). The lack of point‑of‑care testing therefore amplifies the challenge of using oseltamivir judiciously.
Pediatric and Pregnant Populations: Dosing Precision
Children under five often need a liquid suspension, which must be prepared with a precise amount of water. In many settings, clean water is a premium commodity, and the measuring syringes used for dosing may be missing or broken. Pregnant women, meanwhile, must be counseled about the importance of early treatment, but cultural barriers sometimes delay them from seeking care until later in the illness, rendering the drug less effective.
Drug Resistance Monitoring: A Missing Piece
Resistance to oseltamivir has been documented in some influenza strains, especially after prolonged community use. Detecting resistance requires laboratory sequencing, a capability that most low‑resource health systems do not possess. Without surveillance, resistant viruses can spread unchecked, compromising the drug’s future utility.
Comparing Oseltamivir Formulations
| Attribute | Tablet (75 mg) | Pediatric Suspension (6 mg/ml) |
|---|---|---|
| Storage temperature | 15‑30 °C | 15‑30 °C (requires mixing) |
| Shelf‑life | 3 years | 2 years after reconstitution |
| Ease of dosing | Fixed dose for adults | Weight‑based, needs measuring device |
| Water requirement | None | Clean water for reconstitution |
| Common use case | Adult out‑patient | Children < 12 kg |
The table highlights why the tablet form is generally preferred in settings where clean water and measuring tools are scarce. However, when treating infants, the suspension is unavoidable, and clinics must plan for the extra logistics.

Strategic Solutions to Overcome Barriers
- Pre‑positioned stockpiles: Establish regional caches of oseltamivir that can be accessed quickly during outbreaks, reducing reliance on long supply chains.
- Solar‑powered refrigerators: For the few facilities that need to store RDTs or reconstituted suspension, solar units provide a low‑maintenance cooling option.
- Training bundles: Combine drug administration workshops with simple temperature‑monitoring drills, using color‑coded stickers to flag compromised stock.
- Mobile phone inventory apps: Even basic SMS‑based reporting can alert central warehouses to imminent stock‑outs before they happen.
- Task‑shifting: Empower community health workers to dispense oseltamivir after a short rapid‑assessment protocol, extending reach beyond the clinic walls.
These interventions address the biggest oseltamivir challenges by tackling storage, supply, training, and diagnostic gaps in a coordinated way.
Policy and Funding Considerations
Governments and donors often allocate funds to vaccines but overlook antivirals. Advocacy should frame oseltamivir as a cost‑effective pandemic mitigation tool-studies show that each treated severe case can save thousands in hospital costs. Aligning the drug with national influenza preparedness plans ensures that budget lines exist for procurement, storage equipment, and training.
Quick Checklist for Practitioners in Low‑Resource Settings
- Verify storage temperature with a simple heat‑sensitive sticker.
- Confirm expiration dates before dispensing.
- Use calibrated syringes for pediatric suspension; if unavailable, use a documented household measuring cup and record the volume.
- Document onset of symptoms precisely; aim to start treatment within 48 hours.
- Report stock levels weekly via SMS or paper log to regional pharmacy.
- Educate patients on completing the full 5‑day course, even if they feel better.
Frequently Asked Questions
Can oseltamivir be stored at room temperature?
Yes, the tablets are stable between 15 °C and 30 °C for up to three years. Extreme heat above 30 °C can degrade potency, so monitoring is still recommended.
What should I do if the suspension cannot be reconstituted due to lack of clean water?
If clean water is unavailable, prioritize tablet use for patients who can swallow pills. For children who cannot, consider referring them to the nearest facility with safe water or using a boiled‑and‑cooled water source.
How can I identify a possible drug‑resistant influenza strain?
In low‑resource settings, clinical clues such as lack of improvement after 48‑72 hours of treatment may suggest resistance. However, definitive confirmation requires lab sequencing, so report suspected cases to national surveillance programs.
Is it safe to give oseltamivir to pregnant women?
Yes, WHO recommends oseltamivir for pregnant women when influenza is confirmed or suspected, as the benefits outweigh potential risks.
What is the recommended dosage for a 10‑kg child?
The WHO guideline suggests 30 mg twice daily for five days, which translates to 5 ml of the 6 mg/ml suspension per dose.
By understanding the logistical, clinical, and policy layers that affect oseltamivir delivery, health workers can design realistic plans that keep the drug effective even where resources are thin. The goal isn’t just to have the pills on the shelf-it’s to turn them into timely, life‑saving treatment for every patient who needs them.





Eli Soler Caralt
October 21, 2025 AT 15:40In the grand tapestry of global health, oseltamivir emerges as a fleeting comet-brilliant, yet perilously dependent on the fragile scaffolding of infrastructure that many low‑resource clinics simply lack 😅. One might argue that the very notion of "access" is a construct birthed by neocolonial pharmaco‑economics, where the party line is whispered in sterile boardrooms while the bedside murmurs a different requiem. The cold‑chain myth, for instance, is but a metaphor for the brittle promises made by donors, promises that dissolve like sugar in scorching afternoon tea. And yet, the molecule itself, a modest pyrrolidine derivative, dares us to reconsider our preconceptions of what a "simple" pill can achieve. If we fail to honor the therapeautic window of 48 hours, we are not merely missing a dosage; we are betraying a covenant with every vulnerable child whose future hinges on that fleeting therapeutic window. 💊🌍
Eryn Wells
October 21, 2025 AT 16:46Thanks for shedding light on these practical hurdles! 💡 It's vital that we share real‑world solutions-like solar‑powered fridges and SMS inventory systems-so that every clinic, no matter how remote, can keep oseltamivir potent and ready. By fostering collaboration across borders, we can turn isolated challenges into collective triumphs. 🌐🚑
Kathrynne Krause
October 21, 2025 AT 17:53Absolutely love the checklist vibe! 🎉 Let’s rally our community health workers with bright, color‑coded stickers and simple measuring kits-making dosing a breeze even in the hottest villages. When we infuse a dash of creativity into training sessions, the knowledge sticks like glue and the meds stay effective. Keep the momentum going, folks-every small win builds a healthier future! 🌞💪
Chirag Muthoo
October 21, 2025 AT 19:00We appreciate the emphasis on collaborative mechanisms. It is prudent to adopt solar‑based refrigeration where grid reliability is questionable, as such an approach aligns with both cost‑effectiveness and sustainability goals. Additionally, integrating SMS‑based stock alerts can significantly reduce the latency between depletion detection and resupply. These measures, when implemented systematically, will enhance the resilience of antiviral availability in constrained settings.
Angela Koulouris
October 21, 2025 AT 20:06Great points, thank you!
erica fenty
October 21, 2025 AT 21:13Supply chain latency-critical; temperature excursions-dangerous; dosage accuracy-imperative. 📊
Xavier Lusky
October 21, 2025 AT 22:20Remember, the big pharma lobby isn’t just shipping pills; they’re shipping agendas. Every bulk purchase can be a Trojan horse, embedding control mechanisms in our health systems. Beware the “pre‑positioned stockpiles” narrative-it often masks surveillance and market capture. Keep eyes open; the cure can be a conduit for deeper influence.
Ashok Kumar
October 21, 2025 AT 23:26Ah, yes, because the only thing clinicians care about is conspiracy theory, not patients. 🙄 While we’re busy hunting shadows, the actual virus continues to infect. Perhaps we should also doubt the existence of germs altogether?
Jasmina Redzepovic
October 22, 2025 AT 00:33It’s astonishing how often American expertise gets sidelined in these discussions. The US government has already funded the bulk of oseltamivir production, and we’ve pioneered the most efficient distribution algorithms. Other nations should adopt our protocols instead of reinventing the wheel-efficiency is a patriotic duty.
Esther Olabisi
October 22, 2025 AT 01:40Haha, love the confidence! 😆 But let’s remember that sharing best practices works both ways-your “American algorithms” could learn a thing or two from community‑driven distribution models in Africa. Together we can build a hybrid system that’s both high‑tech and grassroots‑friendly. 🌍👍
Ivan Laney
October 22, 2025 AT 02:46When we examine the geopolitical underpinnings of antiviral dissemination, it becomes evident that the narrative surrounding oseltamivir is not merely a tale of pharmacology but a complex saga of power dynamics, economic leverage, and national security interests. The United States, having invested billions in the research and manufacturing pipelines of neuraminidase inhibitors, naturally positions itself as the custodian of global influenza preparedness, a role that some critics argue borders on hegemony. Yet, this custodial claim is bolstered by a robust logistical network that, through strategic pre‑positioning of stockpiles across continents, ensures rapid deployment in the event of a pandemic-a logistical feat rarely matched by other nations. Critics, however, often overlook the fact that such pre‑positioning requires not only substantial capital outlay but also sophisticated inventory management systems that integrate satellite temperature monitoring, automated re‑ordering algorithms, and encrypted communication channels to safeguard against tampering. Moreover, the transition from tablet to pediatric suspension, while seemingly a minor formulation detail, encapsulates a cascade of challenges: the necessity for clean water, calibrated syringes, and caregiver education-all of which are frequently absent in low‑resource settings, leading to suboptimal dosing and, consequently, diminished therapeutic efficacy. It is also worth noting that the stability profile of oseltamivir tablets, stable at ambient temperatures up to 30 °C, offers a distinct advantage over other antivirals that demand strict cold‑chain adherence, thereby reducing the infrastructural burden on remote clinics. Nonetheless, the mere existence of temperature‑sensitive diagnostics, such as rapid influenza tests, introduces an ancillary vulnerability, as these diagnostics themselves rely on refrigeration and quality control mechanisms that many peripheral health outposts lack. In this context, the adoption of solar‑powered refrigeration units emerges as a pragmatic solution, albeit one that necessitates initial capital investment, routine maintenance, and community training to ensure operational longevity. Parallel to these infrastructural considerations, the specter of antiviral resistance looms large; prolonged exposure of viral populations to sub‑therapeutic drug concentrations-often a byproduct of incomplete courses or improper storage-can accelerate the emergence of resistant strains, a phenomenon that underscores the imperative for vigilant pharmacovigilance programs. While high‑income countries possess the laboratory capacity to sequence viral genomes and monitor resistance patterns, low‑resource settings remain largely blind to these trends, creating an information asymmetry that hampers global response efforts. The solution, therefore, lies not solely in the distribution of the drug but in a holistic approach that integrates training bundles, community engagement, and the establishment of regional resistance surveillance hubs, thereby democratizing access to both therapeutic and diagnostic innovations. Ultimately, the success of oseltamivir in curbing influenza morbidity and mortality hinges on a symphony of factors-logistical precision, scientific vigilance, and equitable policy frameworks-that must be orchestrated with both technical acumen and diplomatic finesse.
Kimberly Lloyd
October 22, 2025 AT 03:53Reading through that comprehensive analysis, one is reminded that medicines are not merely chemical compounds but extensions of our collective will to confront suffering. The interplay between temperature, trust, and timely administration mirrors the delicate balance we strive for in our own lives-harmonizing the external conditions with internal readiness. Let us, therefore, treat each oseltamivir tablet as a metaphorical seed, awaiting the right environment to sprout hope in the midst of a viral storm.