When you hear the word biosimilar, you might think it’s just like a generic pill - cheaper, same effect. But that’s not true. Biosimilars aren’t copies of biologics the way aspirin is a copy of aspirin. They’re complex, living medicines made from living cells. And because of that, tiny differences in how they’re made can trigger your immune system in ways the original drug doesn’t. That’s immunogenicity - and it’s why some patients react differently to a biosimilar than they did to the brand-name biologic.
What Is Immunogenicity, Really?
Immunogenicity means your body sees a drug as a foreign invader and makes antibodies against it. These are called anti-drug antibodies, or ADAs. For most drugs, that’s not a big deal. But for biologics - like Humira, Enbrel, or Remicade - ADAs can turn a life-changing treatment into a useless or even dangerous one. If your body starts neutralizing the drug, your inflammation comes back. In rare cases, you might get severe allergic reactions, like anaphylaxis. The scary part? Even fully human proteins can trigger this. Just because a drug looks like your own body’s proteins doesn’t mean your immune system won’t notice small changes. Think of it like recognizing a friend’s face. If they grow a beard, you still know it’s them. But if their eyes change shape, your brain flags it. That’s what happens with biosimilars.Why Biosimilars Are Different From Generics
Generics are simple. They’re small molecules made in a lab, chemically identical to the original. If you have a generic version of metformin, it’s the same compound, down to the last atom. Biosimilars? They’re proteins - thousands of atoms arranged in 3D shapes. They’re made in living cells: Chinese hamster ovary cells, sometimes human cells. Those cells don’t make the same protein every time. Tiny variations in sugar chains (glycosylation), folding, or clumping happen. These aren’t mistakes - they’re natural outcomes of biology. A 2020 study found that differences in sialylation or galactosylation - two types of sugar attachments - affected 15-20% of biologic products. Even a 5% change in the Fc region (a part of the antibody that talks to immune cells) can alter how your body responds. That’s why regulators don’t require biosimilars to be identical. They only need to be highly similar with no clinically meaningful differences. But what’s clinically meaningful? That’s where the debate starts.What Makes One Person React and Another Doesn’t?
It’s not just the drug. It’s you. Your immune system is unique. If you have rheumatoid arthritis, your risk of making ADAs is 2.3 times higher than a healthy person’s. If you carry the HLA-DRB1*04:01 gene variant, your risk jumps 4.7-fold for certain antibodies. Some people’s immune systems are just more alert. Then there’s how the drug is given. Subcutaneous injections - the kind you give yourself - are 30-50% more likely to trigger an immune response than IV infusions. Why? Because your skin is full of immune cells. Every time you inject under the skin, you’re basically waving a flag in front of your immune system. Dosing matters too. If you’re on intermittent therapy - say, every other week - your immune system gets time to recover and notice the drug as foreign. Continuous dosing? Your body learns to ignore it. And if you’re on methotrexate at the same time? That cuts ADA risk by 65% for TNF blockers. It’s like your immune system is distracted.
Manufacturing: The Hidden Variables
Two biosimilars made by different companies can have different stabilizers. One might use polysorbate 80; another, polysorbate 20. That small change can cause protein clumping. And aggregates? They’re a red flag. If more than 5% of the drug forms clumps, immunogenicity risk jumps 3.2 times. Host cell proteins - leftover bits from the manufacturing cells - are another culprit. Above 100 parts per million? ADA rates go up 87%. Even the container matters. Glass vials can leach trace metals. Plastic syringes can absorb proteins. These aren’t big issues in controlled trials, but in real life, patients might switch between vials, brands, or delivery devices. Each switch adds noise to the system.Do We Really See Differences in Real Patients?
Studies give mixed answers. The NOR-SWITCH trial in 2016 followed 481 patients who switched from originator infliximab to its biosimilar. ADA rates went up slightly - 8.5% to 11.2%. But no one got sicker. No more hospitalizations. No loss of response. Then there’s the Danish registry. For adalimumab, the biosimilar Amgevita had a 23.4% ADA rate compared to 18.7% for Humira. Statistically significant. But again - patients still responded to treatment. Their disease stayed controlled. A 2021 study of 1,247 rheumatoid arthritis patients found zero difference in ADA rates between the reference infliximab and its biosimilar CT-P13. 12.3% vs. 11.8%. No difference. On Reddit, patients report both sides. One person said they developed painful injection site reactions after switching to a biosimilar etanercept. Another said they’ve been on three different biosimilars over three years with no issues. So what’s the truth? For most people, the difference is invisible. But for a small group - maybe 5-10% - it matters. And we don’t know who they are until it happens.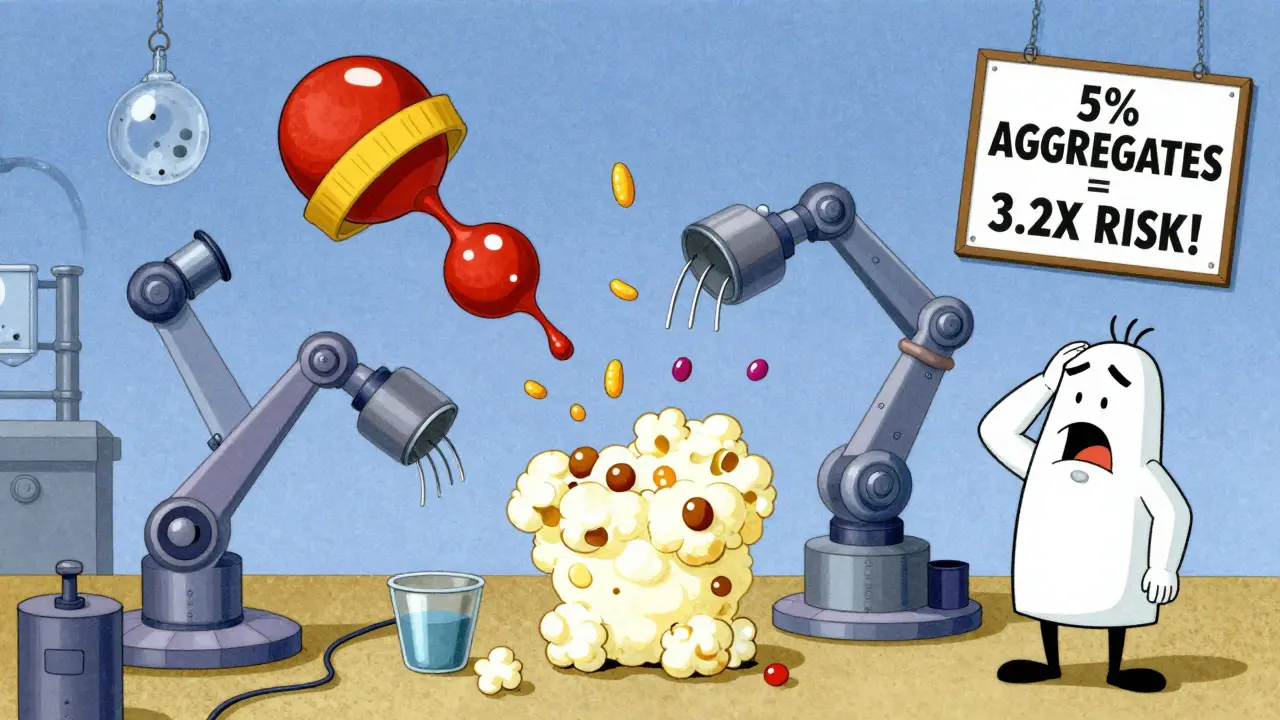
How Do We Measure This?
Testing for ADAs isn’t straightforward. There are dozens of lab methods. Electrochemiluminescence (ECL) assays catch more antibodies than ELISA. But ECL isn’t always used. If one study uses ECL and another uses ELISA, you can’t compare them. That’s why regulators insist on head-to-head comparisons using the same tests. The FDA and EMA require a tiered approach: first screen for any antibodies, then confirm they’re real, then check if they neutralize the drug. Neutralizing antibodies are the real concern - they block the drug from working. Cell-based assays are best for detecting them, even though they’re less precise. Why? Because they mimic how the immune system actually works. But here’s the problem: many real-world studies don’t use the same methods. So when you see headlines saying “biosimilar has higher immunogenicity,” you have to ask: same test? Same patient group? Same timing?What Does This Mean for Patients?
If you’re doing well on a biologic, switching to a biosimilar is usually safe. The data shows that for most people, it works just as well. But if you’ve had allergic reactions before, if you’re on multiple biologics, or if your disease is unstable - talk to your doctor. Don’t assume biosimilar = automatic switch. Ask about the specific product. Ask if they’ve seen reactions with it. Ask if your insurance will cover the original if you need it. And if you switch and suddenly feel worse - more fatigue, more pain, new rashes - don’t brush it off. It might be your immune system reacting. Get tested for ADAs. It’s not routine, but it should be considered.The Future: Better Tools, Fewer Surprises
The next wave of biosimilars will be smarter. Labs are now using advanced mass spectrometry to map protein structures down to the sugar level. By 2027, experts predict we’ll be able to analyze these modifications at 99.5% accuracy. That means manufacturers will be able to control glycosylation so tightly, immunogenicity differences will be nearly eliminated. Meanwhile, researchers at UCSF and others are combining proteomics, glycomics, and immunomics - looking at proteins, sugars, and immune responses all at once. These multi-omics tools could one day predict who’s at risk before they even get the drug. For now, the message is simple: biosimilars are safe for most. But immunogenicity isn’t a myth. It’s a quiet, variable risk - real, measurable, and sometimes meaningful. We’re learning how to manage it. But we’re not done yet.Can biosimilars cause allergic reactions?
Yes, though it’s rare. Some biosimilars have triggered IgE-mediated anaphylaxis, like the case with cetuximab, where a sugar molecule (galactose-α-1,3-galactose) on the drug caused severe allergic responses. While this was with the originator, similar sugar patterns can appear in biosimilars if manufacturing conditions change. Injection site reactions - redness, swelling, itching - are more common and often linked to immune activation, not true allergy.
Are biosimilars always cheaper than the original biologic?
Not always. In the U.S., patent lawsuits and insurance rebates often keep biosimilar prices high. While Europe sees 30-50% savings, U.S. savings for biosimilars average only 15-25%. Sometimes, the original drug is priced lower due to negotiated deals. Always check your out-of-pocket cost - not just the list price.
Should I avoid switching to a biosimilar if I’m doing well?
If you’re stable and feeling good, there’s no medical need to switch. Many doctors recommend staying on your current drug unless cost or access forces a change. Switching isn’t inherently risky, but it’s not risk-free either. The data shows most people do fine, but a small number have trouble. If you’re concerned, ask your doctor about your specific drug and its biosimilar history.
Do biosimilars have more side effects than the original?
Large studies and registries show no consistent increase in overall side effects. Common side effects like headaches, nausea, or fatigue are similar. The only difference that appears in some studies is higher rates of anti-drug antibodies - but even that doesn’t always mean worse symptoms. Clinical outcomes - like disease control, hospitalizations, or flare-ups - are typically the same.
How long does it take for immunogenicity to show up after switching?
It varies. Some patients develop antibodies within weeks. Others take months or even years. The immune system doesn’t react on a schedule. For most biologics, ADA levels peak between 6 and 12 months after starting or switching. That’s why long-term monitoring - at least one year - is recommended after any switch.


Chris & Kara Cutler
January 31, 2026 AT 16:27Melissa Melville
February 2, 2026 AT 11:53Bryan Coleman
February 3, 2026 AT 02:03June Richards
February 4, 2026 AT 12:25Naresh L
February 5, 2026 AT 04:00Donna Macaranas
February 6, 2026 AT 19:34Aditya Gupta
February 8, 2026 AT 11:59Rachel Liew
February 9, 2026 AT 20:38vivian papadatu
February 10, 2026 AT 09:17Bob Cohen
February 10, 2026 AT 09:32Nidhi Rajpara
February 10, 2026 AT 22:18Sami Sahil
February 12, 2026 AT 19:05Jaden Green
February 13, 2026 AT 01:11